Collaboration Strategy
Successful vaccination requires an immune system capable of mounting an effective response to the vaccine. This requires a deeper understanding of underlying principles, including interactions between tumor cells and the immune system. DCprime therefore continues to invest in research into further understanding the condition of the immune system during or after treatment of cancer. We anticipate that disruptive new cancer treatments will be the result of collaborative efforts, so we strive to build out a collaborative network with relevant academic groups, hospitals, and other companies.
Relapse Vaccines Combine Well with Other Therapies
As a post-remission therapy, relapse vaccination could be applied in multiple scenarios and at various stages of treatment of the disease.

DCOne as a Combination Platform for CAR-T and other T Cell-based Therapies
CAR-T therapy has the potency to bring even late-stage patients into remission. Responses are however limited in duration due to the limited life span of CAR-Ts. As with other therapeutic options, residual disease leads often to relapse.

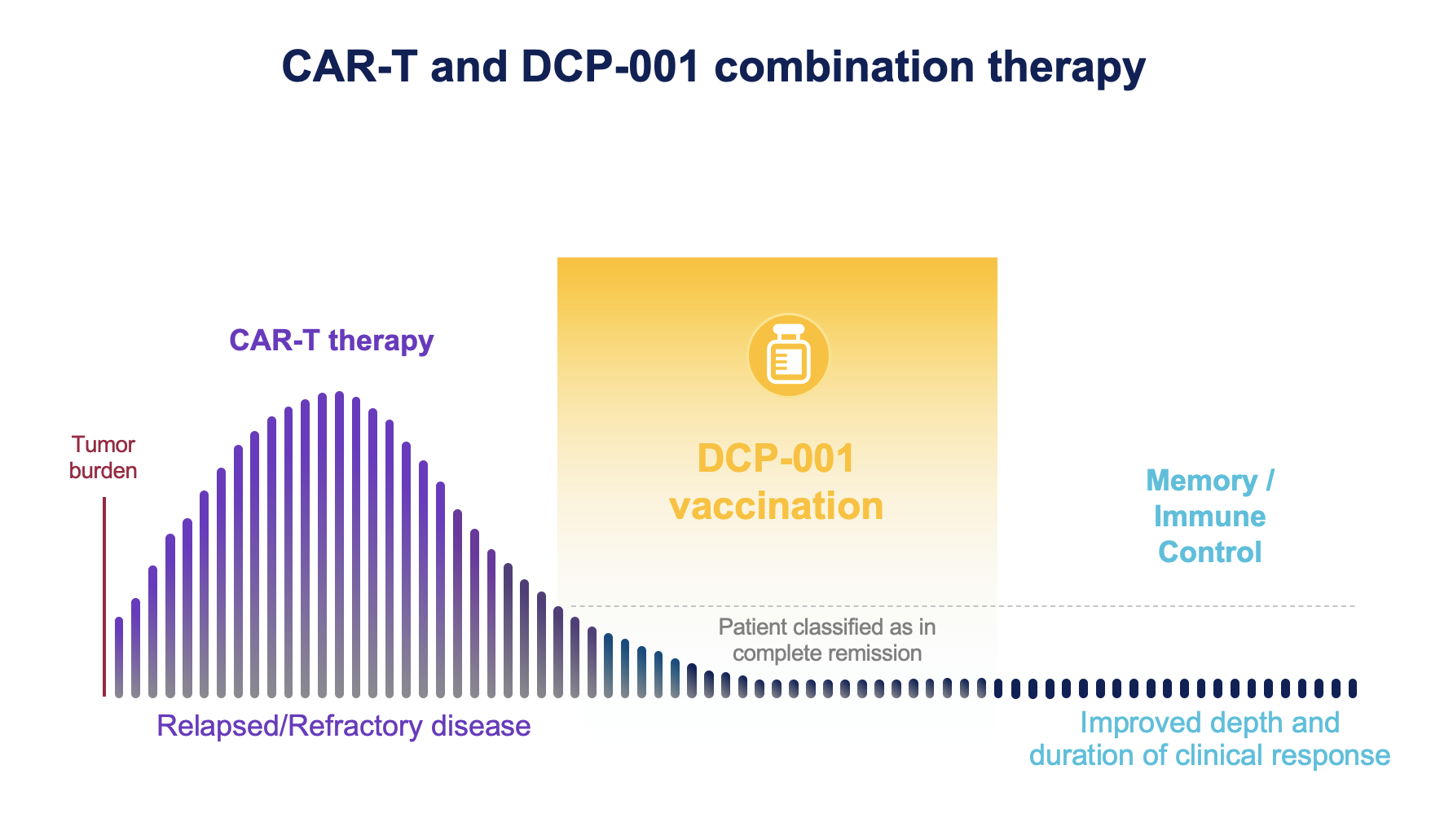
There is emerging evidence that vaccines act synergistically with CAR-Ts. Post-remission, DCP-001 vaccination has the potency to deepen and prolong duration of clinical responses. A combination of both approaches could improve CAR-T function and survival ultimately establishing a broader immune control over residual disease.
Moreover, in the course of DCprime’s R&D development, we demonstrated that the DCOne platform could significantly increase T cell quality by stimulating T cell proliferation, activation and memory formation, and by inducing a higher CD4/CD8 ratio. The same effect is observed in both healthy donors’ and cancer patients’ PBMC. Therefore, the DCOne platform could have valuable benefits when implemented in the CAR-T manufacturing process.
Current Collaborations

In September 2020, DCprime and Glycotope GmbH announced an expansion of their existing partnership through a new research collaboration and licensing agreement. Originally initiated in July 2018, the partnership combines DCprime’s proprietary DCOne® relapse vaccine platform and Glycotope’s highly specific anti-tumor antibody platform with the aim of developing novel immunotherapeutic approaches in oncology. Under the expanded agreement a therapeutic antibody program has been selected from Glycotope’s portfolio which will be further evaluated in preclinical studies to potentially treat a broad range of solid tumors.

In September 2020, DCprime and PCI Biotech announced an extensive research collaboration combining DCprime’s cancer vaccine expertise with PCI’s unique intracellular delivery technology via PhotoChemical Internalisation. The collaboration builds on preclinical proof-of-concept results on a novel cancer vaccination concept based on tumor-independent antigens (TIAs) previously presented by DCprime at the 34th SITC Annual Meeting in November 2019. Within the collaboration, DCprime and PCI Biotech will combine the TIA vaccination concept with PCI Biotech’s technology platforms.

September 2019, DCprime announced the initiation of a research collaboration with the Department of Obstetrics and Gynecology at the University Medical Center of Groningen (UMCG) lead by Professor Hans Nijman MD PhD. The purpose of the collaboration is to design a novel relapse vaccine approach for ovarian cancer and to prepare for a clinical trial in ovarian cancer patients.

