Historically, cancer vaccine development has focused on established, hard-to-treat tumors, with limited success. Established tumors evade the immune system in different ways, including immune suppression, disturbed antigen presentation and an inhibitory tumor microenvironment. Vaccination in this treatment window has led only to limited success.
Relapse vaccines represent a new therapeutic paradigm with the aim to boost the immune system to control residual disease following first treatment. This vaccination window gives the immune system a better chance to regain control, compared to established tumors. DCprime is evaluating the relapse vaccine approach in both blood-borne and solid tumors.
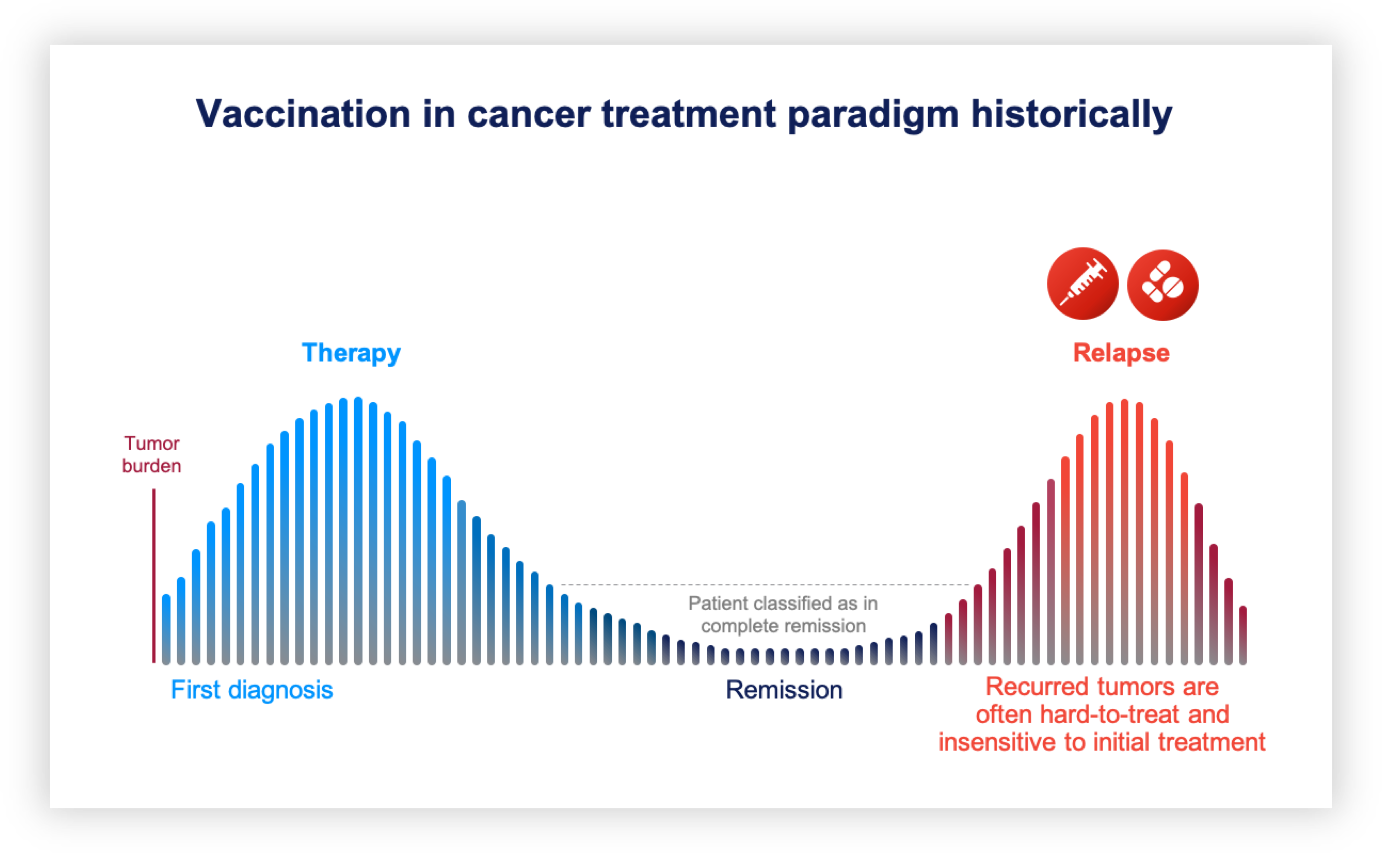

Our relapse vaccines are expected to boost the immune system’s ability to recognize specific tumor antigens to prevent or delay cells carrying these antigens from manifesting as a new tumor. This approach could be combined with other immuno-oncology approaches, launching a highly disruptive combination therapy against hard-to-treat tumors with a high risk of relapse.
DCP-001 – A cancer cell transformed into a vaccine
DCprime’s lead cancer relapse vaccine candidate DCP-001 is generated by transforming a proprietary leukemic cell, DCOne®, into a whole cell-based cancer vaccine. The company most recently presented comprehensive pre-clinical results showing that this transformation leads to an immunogenic shift. Whereas the parental leukemic cells were poorly immunogenic, DCP-001 proved highly immunogenic, making it an attractive cancer vaccine candidate. Following the immunogenic shift, DCP-001 induced the production of a broad range of pro-inflammatory cytokines in peripheral blood mononuclear cells (PBMCs), was able to suppress tumor growth in humanized immunocompetent mice and was efficiently recognized by human antigen presenting cells (APCs) as compared to leukemic cells.

In a separate preclinical study, which was presented at the 2019 CIMT Annual Meeting, DCprime was able to demonstrate that APCs efficiently process DCP-001 through phagocytosis. These in vitro data suggest that in vivo, upon intradermal injection, DCP-001 will induce a strong inflammatory response, and is ingested by both resident and attracted host APCs, which subsequently prime tumor-reactive T cells. These data support the proposed mode of action whereby host APCs present DCP-001 antigens to the host immune system following intradermal vaccination.
